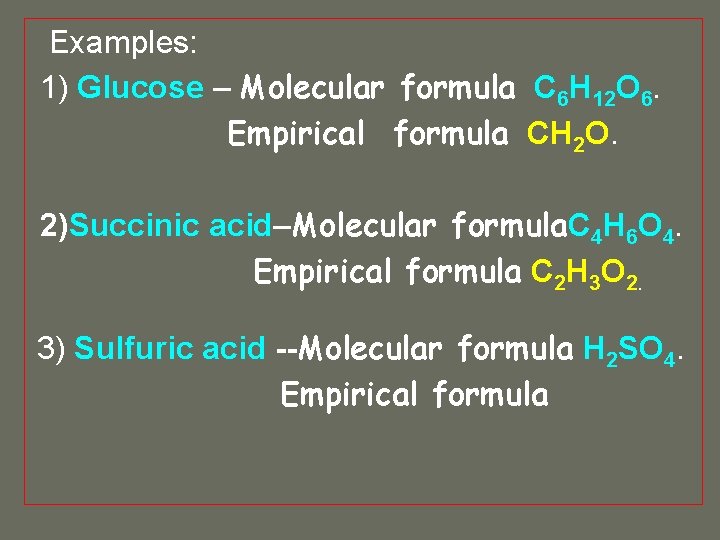
FREE Expert Solution Recall that the empirical formula is the simplest whole number ratio formula of a compound. An example would be CH2O.
 Empirical Formula The Empirical Formula Gives The Lowest
Empirical Formula The Empirical Formula Gives The Lowest
Calculate the percent C in glucose.

Empirical formula for glucose. Empirical formula vs molecular mass definition examples practice problems calculation butane magnesium oxide glucose. The chemical formula of Glucose is C 6 H 1 2 O 6. If you multiply each element in CH 2 O by six you obtain the molecular formula for glucose.
Occasionally as in the case of hydrogen peroxde. Glucose Formula and Facts Glucose is the most abundant monosaccharide in the world and the key energy molecule for Earths organisms. They are different in any case where the molecular formula may be reduced to a smaller whole -number ratio of elements ex.
The Language of Chemistry - Exercise 1 C Page 19 Q 132 Q 131 Q 133. Molecular formula n x Empirical formula Where n is a whole number and is a ratio of molecular mass to empirical formula mass. In liver and muscle tissue UDP-glucose is a direct precursor of glycogen.
It is determined using data from experiments and therefore empirical. Like other sugars glucose forms ismomers which are chemically identical but have different conformations. Empirical formula has been given.
For example the molecular formula for glucose is C 6 H 12 O 6 but the simplest whole-number ratio of the elements in glucose is CH 2 O. Write the empirical formula of the following. This is the empirical formula for glucose which is C6H12O6.
A N2O4 b C6H12O6 c H2O d H2O2 asked May 26 2019 in Chemistry by Aabid 717k points some basic concepts of chemistry. The empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound. Glucose is a commonly used molecule in chemistry.
The formula determined by this method is called the empirical formula or simplest formula. The molecular formula for glucose is C 6H 12O 6. We need to find the mass of one empirical unit.
The empirical formula gives information about the ratio of numbers of atoms in the compound. The molecular formula shows the actual number of atoms. What is its empirical formula.
Advertisement Remove all ads. Acetic acid C 2H 4O 2 has the same empirical formula as formaldehyde CH 2O. It contains 2 moles of hydrogen for every mole of carbon and oxygen.
What is the empirical formula for glucose. The molecular formula of glucose C 6H 12O 6 6 x CH 2O c. An empirical formula for a compound is the formula of a substance written with the smallest integer subscript.
The resulting chain is called cellulose. The structure of glucose C6H12O6 is. The empirical formula is the formula that shows the ratio of atoms.
The molecular formula. For example the molecular formula of glucose is C 6 H 12 O 6 but the empirical formula is CH 2 O. A formula such as this is called an empirical formula.
In lactating mammary gland it is converted to UDP-galactose which is then converted to lactose. This process can be repeated over and over with the chain getting longer and longer. Calculate the empirical formula of molecules through an empirical formula calculator which provides instant results.
Two molecules of glucose can condense by splitting out a molecule of water between them to form cellobiose. Describes how to determine empirical formula from mass data then determine molecular formula using molar mass data. The empirical formula of this molecule is CH2O while the molecular formula is C6H12O6.
They are different for glucose. What is the empirical formula for glucose. This is given in the empirical formula.
The empirical formula for glucose is the simplest ratio of whole numbers components in the compound. All simple sugars have the general formula C n H 2 O n so they all have the same percentage of C H and O. The empirical formula is a basic structure provider while the molecular formula provides.
The empirical formula for glucose is CH 2 O. It transfers a glucose moiety from glucose-1-phosphate to MgUTP and forms UDP-glucose and MgPPi. Empirical Formula Examples Glucose has a molecular formula of C 6 H 12 O 6.
Is there an error in this question or solution. The empirical formula for glucose is CH 2 O. The percent composition of.
While molecular formula is the actual composition of components in the compound. The ration of atoms in glucose is C H 2 O that shows the empirical formula of glucose. You can see that for every one atom of C there are two of H and one of O.
2 oxygen atoms 2 hydrogen atoms 1 magnesium atom 5 atoms. Advertentie Shop Devices Apparel Books Music More.
 What Is The Chemical Formula For Milk Quora
What Is The Chemical Formula For Milk Quora
Magnesium hydroxideMgOH2 commonly called milk of magnesia is both an antacid and a laxative.

Chemical formula of milk of magnesia. The magnesium hydroxide chemical formula is MgOH 2 and its molar mass is 583197 g mol-1. The chemical formula of magnesia is MgO. The molar mass of oxygen is 160 grams per mole.
The generic name for milk of magnesia is magnesium hydroxide and the chemical formula is written as Mg OH 2. Write a balance chemical equation with phases showing the expected reaction that would take place. The most commonly used forms of milk of magnesia are liquid or chewable tablets.
The hydrous magnesium sulfate popularly known as Epsom salts MgSO 4 7H 2 O is used as a laxative. It has an empirical formula of Mg O and consists of a lattice of Mg 2 ions and O 2 ions held together by ionic bonding. Number of Atoms In Each Element Present.
As a suspension in water it is often called milk of magnesia because of its milk-like appearance. Magnesium hydroxide is not very soluble in water K sp 18 x 10 -11. The chemical name of milk of magnesia is magnesium hydroxide hope so my answer is useful _.
How much magnesium hydroxide should be in a 5 mL sample of milk of magnesia. Advertentie Shop Devices Apparel Books Music More. Milk of magnesia is generally sold in liquid form and is an inorganic compound that looks similar to milk hence the name.
Magnesium hydroxide is an inorganic compound with the chemical formula MgOH2. Free UK Delivery on Eligible Orders. The chemical formula shows that milk of magnesia is made of one magnesium atom two oxygen.
Milk of Magnesia Mint-O-Mag Magnesia Magma Magnesium Hydrate. The name milk of magnesia comes from the cloudy or milky appearance of the magnesium hydroxide in water. The molar mass of hydrogen is 10 grams per mole.
Magnesium oxide Mg O or magnesia is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium see also oxide. Milk of magnesia works by bringing water into the intestines to aid in the movement in the intestines according to WebMD. MOM is a magnesium hydroxide suspension.
The fine particle structure formed in. Unlike other magnesium hydroxide products the MgOH2 in milk of magnesia is produced from basic magnesium carbonate. The solid mineral form of magnesium hydroxide is known as brucite.
You can choose any antacid that is primarily magnesium hydroxide Mg OH 2. Magnesium hydroxide also known as milk of magnesia or magnesium dyhydroxide is an inorganic base used as antacids and laxative. It is available over the counter as well as by prescription.
Milk of Magnesia is a suspension of Magnesium HydroxideMilk of MagnesiaDouble-Strength Milk of Magnesiaand Triple-Strength Milk of Magnesia contain not less than 900percent and not more than 1150percent of the labeled amount of MgOH 2the labeled amount being 80160and 240mg of MgOH 2 per mLrespectivelyIt may contain not more than 005percent of a volatile oil or a blend of volatile. As an antacid milk of magnesia works to reduce stomach acid and as a laxative it works to speed up the rate of excretion via the bowels. Milk of magnesia is often used as a laxative for the treatment of constipation.
What is the empirical for milk of magnesia. The molar mass of this chemical compound is equal to 583197 grams per mole. Magnesium hydroxide is an inorganic compound with the chemical formula MgOH2.
What is the mass of 32 moles of milk of magnesia. As a suspension in water it is often called milk of magnesia because of its milk-like appearance. The molar mass of magnesium is 243 grams per mole.
The chemical formula for milk of magnesia is Mg OH2. Properties of Milk of Magnesia The chemical formula of milk of magnesia can be represented as Mg OH 2. Chemical Formula of Milk of Magnesia or Magnesium Hydroxide is Mg OH2.
Free UK Delivery on Eligible Orders. Magnesium hydroxide suspended in water is known as milk of magnesia and it is sold under the name brucite. The best-known medical compounds are milk of magnesia or magnesium hydroxide which is used as an antacid or as a mineral supplement to maintain the bodys magnesium balance.
Milk of Magnesia MOM is a common antacid. Under standard conditions this compound exists as a white solid that does not have any characteristic odour. Milk of magnesia has the chemical formula MgOH2.
See full answer below. 3 g I think its D. The name dates back to Charles Henry Phillips.