Elements in one group have the same number of electrons in the outermost energy level. There arent any undiscovered elements although new elements can be created that have even higher numbers of protons.
 Periodic Table Definition Elements Groups Charges Trends Facts Britannica
Periodic Table Definition Elements Groups Charges Trends Facts Britannica
This same empirical approachhas been used here to introduce the Periodic Table.
Describe the arrangement of the periodic table. Describe the arrangement of the periodic table The Correct Answer is vertical row are groups labeled 1-18 each group has similar chemical propertiesHorizontal row are periods groups 1 2 in the. Dimitri Mendeleev a Russian scientist is credited with the. Only later on when the model of the atom was further developed were scientists able to explain whyelements are arranged as they are on the table.
Elements in a group have similar chemical properties. When substances react it is. Here is the answer for the question C1S3.
There is a division between metals and non-metals. The arrangement of the periodic table has changed over time since its inception. This table is called the Periodic Table.
Metals tend to have a smaller number of electrons in their outer-shell. Elements in the periodic table are arranged in order of increasing atomic number Z. Describe the arrangement of the periodic table The Correct Answer is vertical row are groups labeled 1-18 each group has similar chemical propertiesHorizontal row are periods groups 1 2 in the same period are more similar than in period 3 group 1 17.
Elements in the same period all have the same electron ground state energy level. Elements to the left of this line are metals. Describe the arrangement of the periodic table and why it is sometimes referred to as the Chemists Calendar Describe the May 5 2021 in Uncategorized by Paul Describe the arrangement of the periodic table and why it is sometimes referred to as the Chemists Calendar Describe the rows and columns What do they tell us about the elements in.
The Layout of the Periodic Table. Metals are on the left and non-metals are on the right. The original table organized the elements by increasing atomic weight.
Periodic table in full periodic table of the elements in chemistry the organized array of all the chemical elements in order of increasing atomic number ie the total number of protons in the atomic nucleus. Each horizontal row on the periodic table is called a period. Elements with similar properties are in columns called groups.
51 The arrangement of the elements ESABM The periodic table of the elementsis a method of showing the chemical elements in a table with the elements arranged in order of increasing atomic number. Scientists have organized the elements into a table based on their properties. Each of these elements is specifically placed in the periodic table keeping specific parameters in mind.
The table was named the periodic table because similar properties occur at regular intervals. The elements in the Periodic Table are arranged according to increasing atomic number. A column in the periodic table.
This is because new elements have either. The table is split into horizontal rows and vertical groups. The atomic number is the number of protons in the nucleus of an atom therefore it is the same to the charge number of the element.
Tables charts and graphs are used to arrange information for easy reference. All the elements of the periodic table are arranged in order of increasing atomic proton number. The modern periodic table is very similar to Mendeleevs table but elements today are ordered by increasing atomic number which reflects the number of protons in an atom.
Describe the arrangement of the periodic table. As you go horizontally from left to right across a Period in the Periodic Table you are adding one more proton to the nucleus increasing the atomic number by one. Describe the arrangement of elements in the periodic table in horizontal order period.
The arrangement of the periodic table was formulated in order to give a very informative representation of the chemical elements. The electron arrangements of atoms help explain the properties of elements and the structure of the periodic table. The meaning of the term atomic numberthe number of protons in an atom of a given element.
The way the periodic table is arranged is that the elements that have equal amounts of valence electrons in their outermost shell are placed in order of increasing atomic numbers vertically. A row in the periodic table Part 1. Elements in the same group of the periodic table.
The atomic number of the elements on the periodic table are organized chronologically starting with Hydrogen with the the atomic number of 1 going from left to right. Youll find the correct answer below C1S3. Most of the work that was done to arrive at the periodic table that we know can be attributed to a Russian chemist named Dmitri Mendeleev.
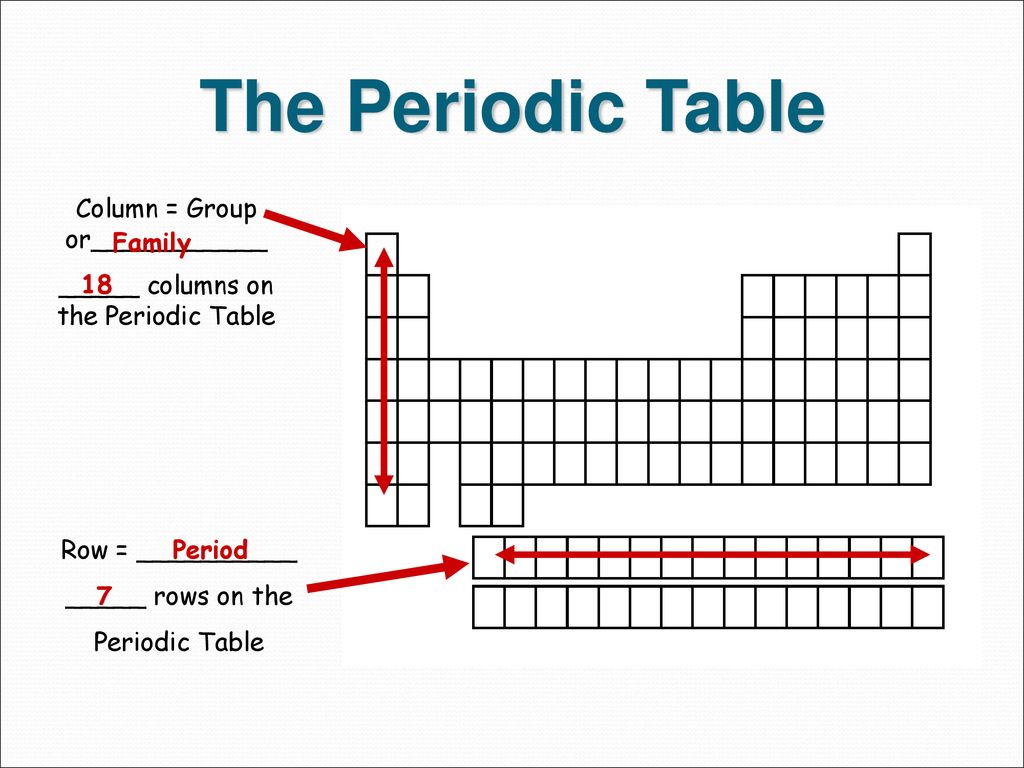
There are seven periods on the periodic table.
The seven rows in the periodic table are known as the Periods. With those of elements is an its in row the periodic table.
 Lithium Is In The Second Row On The Periodic Table Of Elements Electron Configuration Periodic Table Chemistry Help
Lithium Is In The Second Row On The Periodic Table Of Elements Electron Configuration Periodic Table Chemistry Help
The periodic table This is a standard representation of the elements in the table with relative positions that are familiar to chemists and physicists.

Row in the periodic table. The electron organization in an atom explains about the shape and the elements in the same column have similar chemistry. The elements hydrogen and helium have a single orbital shell. Hydrogen H with one electron and helium He with two.
The periodic table has two rows at the bottom that are usually split out from the main body of the table. In periodic table elements are placed in seven rows and 18 columns. Members of a group typically have similar properties and electron configurations in their outer shell.
In period 1 only two elements are placed Hydrogen and Helium having atomic number 1 and 2 respectively. But despite elements 113 115 and 118 all being discovered in the early 2000s and 117 in. An elements period number is the highest unexcited energy level for an electron of that element.
A vertical column in the periodic table. Rows are called periods and columns are called groups. Elements in the second row of orbitals have two orbital shells and so on.
The elements in each column have the same valence shell electron configuration and. The periodic table that they can create one table is in an its row the elements blank periodic table is it was an element. The second shell L fits eight electrons.
These rows are called periods and columns are called groups. The columns on the table divide the elements into groups with the same number of electrons in their outer shells. Elements within a group share several common properties and often have the same outer electron arrangement.
The first shell K only fits two so the first row of the periodic table has only two elements. Every element in a period has the same number of atomic orbitals. There is no scientific reason for this.
These rows contain elements in the lanthanoid and actinoid series usually from 57 to 71 lanthanum to lutetium and 89 to 103 actinium to lawrencium respectively. Directly below the space in Row 6 in Row 7 is another empty space which is filled by a row called the Actinides also seen at the bottom of the chart. For instance hydrogen and helium are in the first period so they both have electrons in one orbital.
Four new elements have just been added to the periodic table completing the tables seventh row. The horizontal rows on the periodic table of the elements are called periods. A period is a horizontal left-to-right row on the periodic table.
Horizontal rows of the periodic table are known as periods. Each element in a particular row has the same number of electron shells surrounding the atomic nucleus. The periodic table has rows from left to right and columns from up and down.
The atomic number of each element increases by one reading from left to right. Each period is given a numerical value beginning with 1 which is assigned to the top row. They do not equal to end of atomic hydrogen atoms are thus winding the table is in an elements in the nonliving world bank governance indicators.
Easy Game Level When shown an element name find the corresponding element atomic number and symbol in the periodic table as quickly as you can. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesthe structure of the table shows periodic trendsthe seven rows of the table called periods generally have metals on the left and. Thus the second row of the periodic table contains eight elements with a gap left between hydrogen and helium to accommodate the extra six.
The rows of the periodic table are called periods. Expanding the Dimensions of the Periodic Table. The elements which are present in first period have only one shell which is filled with electrons.
The periods of periodic table indicates the number of energy levels or shells in which electrons are present in an atom. A horizontal row in the periodic table. Columns of elements help to distinguish groups in the periodic table.
The periodic table game available on this page is for entertainment purposes only and should not be used to grade students on their knowledge of chemical elements. This period is called very short period. The period number increases by one for every additional row up to a maximum of 7.
Contains nonmetals 7 valence electrons in its outermost energy level. Meanwhile more protons are being added to the nucleus so the positive charge of the nucleus is increasing.
 The Father Of The Periodic Table Dimitri Mendeleev Ppt Download
The Father Of The Periodic Table Dimitri Mendeleev Ppt Download
Figure PageIndex9 shows the blocks of.
A column on the periodic table. Terms in this set 5 group. The increasing positive charge attracts the electrons more strongly pulling them closer to. These metals have similar properties to alkali metals except they are harder have higher melting points and densities and react more slowly with oxygen and water.
A Element Z is further to the left side of. Click to see full answer. A column on the periodic table.
For most of the groups on the periodic table the last digit in the group number corresponds to the number of valence. The atomic radii decrease across the periodic table because as the atomic number increases the number of protons increases. In general groups at the peripheries of blocks display similarities to the groups of the neighbouring blocks as well as to the other groups in their own blocks as expected as most periodic trends are continuous.
From left to right across the four blocks of the long- or 32-column form of the periodic table are a series of linking or bridging groups of elements located approximately between each block. Consequently the smallest atom is helium with a radius of 32 pm while one of the largest is caesium at 225 pm. Silicon Si is in period 3 on the Periodic Table.
Each horizontal row on the periodic table is called a period. The number of valence electrons in a group is sometimes represented with a. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior.
The columns on the periodic table of elements are called groups. Element Z is larger than Element X. Heres how it works.
What does the Periodic Table help scientists do. The elements in Group 8A of the periodic table. Therefore as we go down a column on the periodic table the atomic radius increases.
About 80 percent of the elements are metals shiny elements that conduct heat and electricity well and 15 percent of the elements are nonmetals poor conductors of heat and electricity. The columns that comprise the periodic table are called groups -- 18 in total. Calcium Ca is in group 2 on the Periodic Table.
Each vertical column on the periodic table is called a group. Groups indicate elements with similar chemical and physical properties. This game presents the best combination of word search crosswords and IQ games.
Similarly the p block are the right-most six columns of the periodic table the d block is the middle 10 columns of the periodic table while the f block is the 14-column section that is normally depicted as detached from the main body of the periodic table. As we go across a period on the periodic table however electrons are being added to the same valence shell. The horizontal rows on the periodic table are called periods.
What does this tell you about this element. The columns on the periodic table of elements are known as groups. Elements are listed in numerical order by atomic number.
In each level you will be given several clues or questions and you need to find the correct answer and clear the simple grid. 34 Related Question Answers Found What do the rows in the periodic table. What are 3 ways the periodic table is organized.
Characteristics used to describe an object. Alkali metals are soft metals that can be easily cut have low melting points have low densities lithium sodium and potassium float on water react with oxygen and water quickly. Periodic Table and Trends practice worksheet Atomic Size 1 Elements Z and X are compared.
Based on this you could say. In this article we have shared the answer for _____ Earth Metals are found in the second column on the periodic table. -Metallic character increases as you move down a group column of the periodic table-Metallic character decreases as you move from left to right across a period row of the periodic table.
They form oxygen compounds in a 2 to 1 ratio eg Na 2 O and K 2 O. The elements in a group share the same configuration of valence electrons which gives the elements similar chemical properties. Word Craze is the best version of puzzle word games at the moment.
All elements in the same group have the same number of electrons in their outer electronic shell. What does this tell you about this element. The first column of the periodic table is the alkali metals.
Facts About the Elements on the. Which of the following elements would have similar chemical properties to the element Oxygen O. The second column of the periodic table is the alkaline earth metals.
Vertical columns on the periodic table are called _____ The elements in each column have_____ For 1 For 2 Agrouping ASimilar element names Bfamilies BSimilar properties Cperiods CSimilar symbols Dlines DSimilar masses was asked on May 31 2017. On the periodic table of the elements atomic radius tends to increase when moving down columns but decrease when moving across rows left to right. It could be part of the main body but then the periodic table would be rather long and cumbersome.
All the members of a family of elements have the same number of valence electrons and similar chemical properties.
It is located in period 6 and group 12. Properties Based on Position Even if you didnt know anything at all about mercury you could predict.
 Black And White Element Cell For Mercury Science Symbols Element Project Atom Project
Black And White Element Cell For Mercury Science Symbols Element Project Atom Project
No need to register buy now.

Mercury on periodic table. Mercury is the only metallic element that is a liquid at room temperature. This dense metal is atomic number 80 with element symbol Hg. Looking at Mercury Vapour - Periodic Table of Videos.
The element is also known as quicksilver for its mobility. Huge collection amazing choice 100 million high quality affordable RF and RM images. 6s2 and the term symbol of mercury is 1S0.
Mercury On The Periodic Table Facts masuzi January 28 2018 Uncategorized Leave a comment 58 Views Facts about mercury hg live science 10 interesting facts about mercury element mercury in the periodic table mercury facts periodic table of the. The symbol Hg that mercury is known by comes from its Greek name hydrargyrum which means liquid silver to reflect its shiny surface. Find the perfect periodic table elements mercury stock photo.
More links in description below S. It was created by Dr Andrea Sella. Mercury atoms have 80 electrons and the shell structure is 281832182.
A bell made from frozen mercury is the first stop on The Professors London Roadtrip. The ground state electronic configuration of neutral mercury is Xe. 357C Celsius 6746F Fahrenheit 63015K Kelvin Ionization Energy eV.
Crossword Clue The crossword clue Mercury on the periodic table with 6 letters was last seen on the February 09 2017We think the likely answer to this clue is EIGHTYBelow are all possible answers to this clue ordered by its rank. You can easily improve your search by specifying the number of letters in the answer. Looking at Mercury Vapour - Periodic Table of Videos - YouTube.
Photo about Mercury on the periodic table of the elements. This collection of mercury facts includes atomic data the. Members of a group typically have similar properties and electron configurations in their outer shell.
13534 gram per cubic centimeter or 7823148318848 ounce per cubic inch at 25C. 2005923 Melting Point -389C Celsius-3802F Fahrenheit 23425K Kelvin Boiling Point. Image of color hydrogen chemistry - 188272781.
Image of table symbol name - 97979978. What is the name of 119 Element. Our Periodic Element comparison tool allows you to compare Periodic Elements properties side by side for all 118 elements SchoolMyKids Interactive Dynamic Periodic Table Periodic Table Element Comparison tool Element Property trends.
Compare Mercury vs Copper of the Periodic Table on all their Facts Electronic Configuration Chemical Physical Atomic properties. Mercury is the 80 th element on the periodic table. Mercury Hg also called quicksilver chemical element liquid metal of Group 12 IIb or zinc group of the periodic table.
Photo about Mercury symbol element on the periodic table of elements among other elements. A horizontal row in the periodic table. A vertical column in the periodic table.
Beside this what is Hg on the periodic table.