In each ionic crystal the cations and anions are isoelectronic with inert gas configuration. Atomic Radius of Chemical Elements.
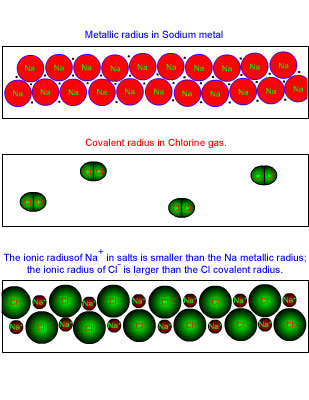 Foundations To Chemistry Adapted From Chemistry Matter And The Universe
Foundations To Chemistry Adapted From Chemistry Matter And The Universe
Three isotopes exist hydrogen with one proton in the nucleus deuterium with a proton and neutron and tritium with one proton and two neutrons in the nucleus.
Ionic radius of hydrogen. There are several other ways ways to define radius for atoms and ions. In the case of Hydrogen the ionic radius is 154 1 Å. The hydrogen atom consists of a proton and an electron with an atomic radius of 037 Å.
K - 2 8 8 Cl - - 2 8 8 Ar type. The following web interface allows listing and comparison of ionic and crystal radii with different coordination and charge states. Both electrons of the hydrogen ion have the same energy level and they dont shield the charge of the core from one another.
The chemical symbol for Hydrogen is H. The Aufbau and Pauli exclusion principles and the assumption of hydrogenic orbitals are insufficient to explain the structure and stability of ceH-. Pauling has calculated the radii of the ions on the basis of the observed internuclear distances in four crystals namely NaF KCl RbBr and CsI.
Values for ionic radius are difficult to obtain and tend to depend on the method used to measure the size of the ion. VdW radius nm ionic radius of X-nm F. Calculation of ionic radii.
Follow the appropriate hyperlinks for literature references and definitions of each type of radius. Hydrogen forms the only cation that has no electrons but even cations that unlike hydrogen still retain one or more electrons are still smaller than the neutral atoms or molecules from which they are derived. Hydrogen forms an hcp solid at low temperatures melting at - 2592 C and boiling at - 2527 C.
The hydrogen anion with its loosely held two-electron cloud has a larger radius than the neutral atom which in turn is much larger than the bare proton of the cation. Although neither atoms nor ions have sharp boundaries it is useful to treat them as if they are hard spheres with radii. Roughly about the same size as an oxide ion.
Ionic radius may be measured using x-ray crystallography or similar techniques. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. In particular it helps to drop the assumption that the electrons occupy the same spatial orbital and differ only according to.
Suggestions as to how the scope and content of the database can be. This article in nature disproves the above and states. Covalent radius is the.
Atomic Radius of Hydrogen Van der Waals radius. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The bond length in HH is.
An ionic radius is one-half the distance between the nuclei of two ions in an ionic bond. Na - 2 8 F- - 2 8 Ne type configuration KCl crystal. The ionic radius of an H X ion is 1342pm which is slightly larger than the radius of F X I I 1285pm and almost the same as the radius of O X 2 I I 135pm.
A typical value for an ionic radius would be from 30 picometers pm and equivalent to 03 Angstroms Å to 200 pm 2 Å. The ionic potential valence divided by ionic radius may be used 1 for classification of metals into acids and bases 2 for ranking of strength in that classification and 3 for prediction of coal ash melting behavior and viscosity from the chemical composition. All values of radii are given in picometres pm.
The role of an acid is that of a complex ion former with anions provided by. In this way the sum of ionic radii of a cation and an anion can give us the distance between the ions in a crystal lattice. All atoms ions have an ionic radius even Hydrogen.
Welcome to the database of ionic radii. A quick analysis of the alkali metal hydride crystal structures and using the Shannon-Prewitt ionic radii for the metals gives an average 13 angstrom 130 pm radius for H-. The atomic configuration of the atom is 1s 1 and it has an ionic radius of 0208 nm.
Ionic radius r ion is the radius of an ion regardless of whether it is an anion or a cation. Ok so what is the ionic radius of a Hydrogen ion. In principle Vana der Waals radius is half the minimum distance between the nuclei of two atoms.
TRANSITION 72 126 Large cation atomic radius Because of the strong oxygen-hydrogen bond the hydrogen is not likely to leave and bond with another water molecule Overall Decreasing Ionic Radius Strong bonds because the electrons are not as strongly attracted to the oxygen. Hydrogen ions are formed either by accepting an extra electron from an atom which is more electropositive than the hydrogen or donating the only electron to the atom which is more electronegative than the hydrogen. 88 sor Hydrogen.
Database of Ionic Radii. Ionic Radius of Hydrogen H Discovery Color Uses.
Ionization Energy And Electronegativity
Why Is The Ionic Radius Of Lithium 1 Ion Greater Than That Of Magnesium 2 Ion Quora
 More Bonding Quick Overview Of Ionic Bonding Metallic Bonding Hydrogen Bonding Quick Overview Of Ionic Bonding Metallic Bonding Hydrogen Bonding Ppt Download
More Bonding Quick Overview Of Ionic Bonding Metallic Bonding Hydrogen Bonding Quick Overview Of Ionic Bonding Metallic Bonding Hydrogen Bonding Ppt Download
 6 20 Ionic Sizes Chemistry Libretexts
6 20 Ionic Sizes Chemistry Libretexts

How To Find Ionic Radius Yahoo Answers
