The absolute value of the unit of mass is obtained by dividing the unit by the Avogadro constant NA. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element.
 2011 Core Ib Chemistry Topic 02
2011 Core Ib Chemistry Topic 02
Thus since the atomic mass of iron is 55847 amu one.
What is an amu in chemistry. An atomic mass unit is defined as a mass equal to one twelfth the mass of an atom of carbon-12. Atomic Mass unit ChemistryWhat is Atomic mass unit AMUAn atomic mass unit symbolized AMU or amu is defined as precisely 112 the mass of an atom of. As a form of measurement the AMU is used to indicate the so-called mass of an atom or molecule.
1 amu is equal to the mass of 112 th the mass of C-12 atom. The amu is the unit that is used to express the atomic mass of a chemical element. For example one atom of helium-4 has a mass of 40026 amu.
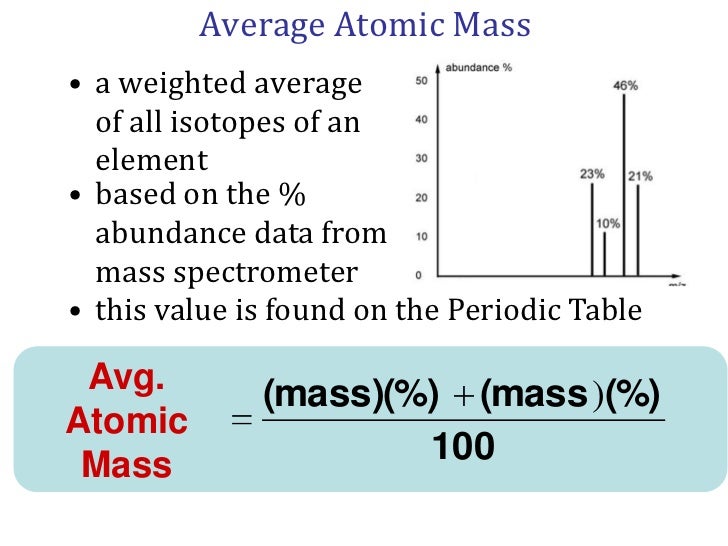
It is a unit of mass used to express atomic masses and molecular masses. The term amu stands for atomic mass unit and is used for the measurements of very small substances such as atoms. Definition for Atomic Mass Unit AMU A mass unit that is exactly 112th the mass of a carbon 12 C12 atom approximately 167E-24g.
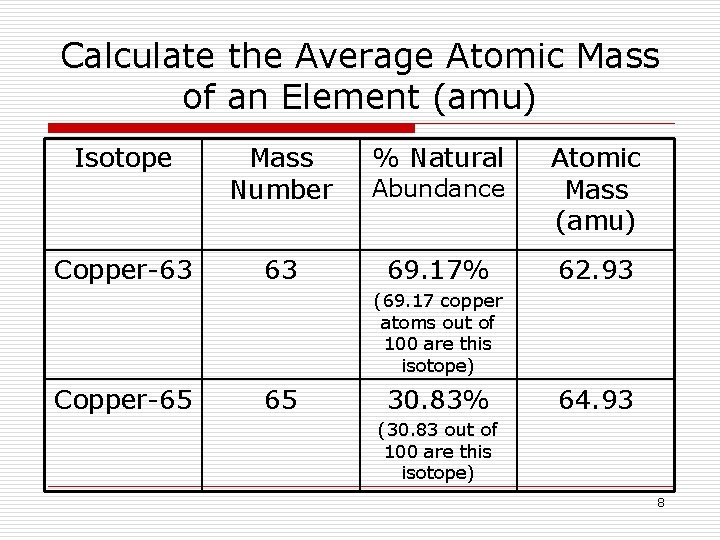
The average atomic mass sometimes called atomic weight of an element is the weighted average mass of the atoms in a naturally occurring sample of the element. The way the u is chosen ensures that all core and atom masses are multiples of 1pm 01 u. Hydrogen for example is the first element.
Hence the actual mass in the amu of an atom of an element will be approximately equal to its mass number. The mass of any isotope of any element is expressed in relation to the carbon-12 standard. Astream of humid air containing 150 mole h_2o v and the balance dry air is to be humidified to a water content of 100mole h_2o.
Chemistry AMU abbreviation meaning defined here. The mass of any isotope of any element is expressed in relation to the carbon-12 standard. Atomic mass unit AMU or amu An atomic mass unit symbolized AMU or amu is defined as precisely 112 the mass of an atom of carbon-12.
In chemistry an atomic mass unit or AMU is a physical constant equal to one-twelfth of the mass of an unbound atom of carbon-12. The mass of the electron is negligible compared to the mass of these particles. The actual masses of protons and neutrons are about 1 amu.
Definition of Atomic Mass Unit AMU One twelfth of a mass of an atom of the carbon-12 isotope. For this purpose liquid water is fed through a flowmeter and evaporated into the air stream. The u amu is the old unit name is 112 of the weight of an 12ce C atom.
Atomic mass units are described as a unit of measurement for atoms and molecules just like the mass of a person may be expressed in pounds or kilograms. The main difference between amu and grams is that amu is used to express the mass in atomic level whereas gram is used as a metric unit of mass. The designation is 1 amu amu atomic mass unit.
Average masses are generally expressed in unified atomic mass units u where 1 u is equal to exactly one-twelfth the mass of a neutral atom of carbon-12. The carbon-12 C-12 atom has six. For example one atom of helium-4 has a mass of 40026 amu.
When there are more protons and neurons the mass and the AMU will also be larger. The u is a count of nucleons in an atom. An atomic mass unit is defined as a mass equal to one twelfth the mass of an atom of carbon-12.
Created by Sal Khan. Hence the atomic weight of an element is approximately equal to its mass number. When talking about the mass of a particular molecule for example it simply refers to the total number of protons and neutrons.
Amu is short term for atomic mass unit. The AMU is a standard unit that measures the mass of atoms or molecules using the sum of protons and. Get the top AMU abbreviation related to Chemistry.
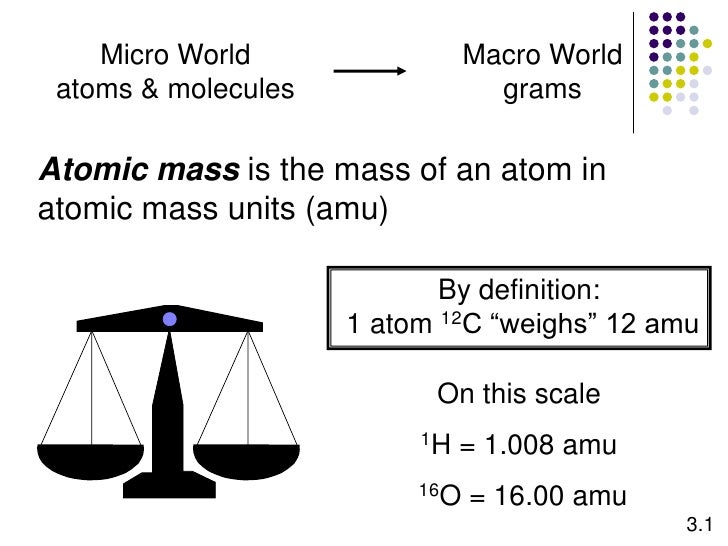
What does AMU stand for in Chemistry. An atom of sulfur-32 has a mass of 31972 amu. By definition one atom of carbon-12 is assigned a mass of 12 atomic mass units amu.
In chemistry AMU stands for atomic mass unit. It is used in chemistry to calculate the mass of elements. A unit used for stating atomic and formula weights.
The atomic mass is useful in chemistry when it is paired with the mole concept. It can be defined as. The only available calibration data for the flowmeter are two points scribbled on a.
The relative mass number of an element thus indicates how many times heavier the atoms of the element are on average than the atomic mass unit.
 Entiteta Gooey Smisel Atomic Mass Unit Susiessewingsensations Com
Entiteta Gooey Smisel Atomic Mass Unit Susiessewingsensations Com
 Ch 1 06 The Arithmetic Of Chemistry
Ch 1 06 The Arithmetic Of Chemistry
 Chemistry Ii Video 3 2 Amu And Molar Conversions Youtube
Chemistry Ii Video 3 2 Amu And Molar Conversions Youtube
 Mass Relationship In Chemical Reaction Ppt Video Online Download
Mass Relationship In Chemical Reaction Ppt Video Online Download
 Average Atomic Mass Video Khan Academy
Average Atomic Mass Video Khan Academy
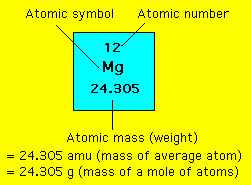 Quantitative Chemistry Mole And Avogadro
Quantitative Chemistry Mole And Avogadro
 Chapter 4 Atoms And Elements Ppt Video Online Download
Chapter 4 Atoms And Elements Ppt Video Online Download
 Pin By Makaylah Randolph On Chemistry Education Atomic Mass Unit Chemistry Education Science Literacy
Pin By Makaylah Randolph On Chemistry Education Atomic Mass Unit Chemistry Education Science Literacy