A molecule lying inside the liquid is surrounded by other molecules and so is attracted equally in all directions. Intuitively it keeps a barrier between foreign materials and liquid as well as this is the force that holds the liquid molecules bind together.
/139802493-56a12f615f9b58b7d0bcde91.jpg) Surface Tension Definition In Chemistry
Surface Tension Definition In Chemistry
As a result the surface area is increased which favours lipase activity on lipids.

Surface tension definition chemistry. Surface tension is responsible for the curvature of the surfaces of air and liquids. That means a drop of water will want to have the smallest possible surface area. Surface tension is a fundamental property of the surface of liquid.
According to the definition of surface tension it is the phenomenon that occurs when the surface of a liquid is in contact with another phase it can be a liquid as well. Liquids that have strong intermolecular forces like the hydrogen. Surface tension is the energy or work required to increase the surface area of a liquid due to intermolecular forces.
Surface tension is the amount of energy required to increase the surface of the liquid by unit area. Liquids tend to acquire the least surface area possible. The surface tension of a liquid results from an imbalance of intermolecular attractive forces the cohesive forces between molecules.
A molecule in the bulk liquid experiences cohesive forces with other molecules in all directions. Hello guys today we are going to discuss term Surface Tension its definitionunits and Factors in which surface Tension depends. Let consider these two setups a and b.
Surface tension is a property of liquid which arises due to the fact that the molecules of the liquid at the surface are in different situation than those in the interior of the liquid. Gasoline or solutes in the liquid eg. Because bile salts reduce the surface tension of lipids and thus assist emulsification.
The surface tension of a liquid is a measure of the elastic force in the liquids surface. It is usually measured in dynes per centimeter. The surface tension is an important property for many processes and physical phenomena.
The SI unit is Newton per meter. Surfactants like detergent each solution exhibits differing surface tension properties. In other words it is also the property of the liquid surface that resists force.
This topic is of chapter. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. The shape that has the smallest possible area for.
Because of this property certain insects can stand on the surface of water. However experimental data are usually scarce and in limited temperature ranges. Page 3 of 13 Surface tension is defined as the force acting over the surface of a solution per unit length of the surface perpendicular to the force.
The surface tension is also arguably the most important of the inhomogeneous fluid properties. Surface Tension The molecules in the bulk of the liquid attracted equally from all the sides but the molecules of liquid present in the surface are attracted towards interior because there are no molecules above the liquid surface. Water has a high surface tension because the water molecules on the surface are pulled together by strong hydrogen bonds.
So surface molecules experience resultant downward pull and behave as a stretched elastic membrane. This phenomenon can be observed in the nearly spherical shape of small drops of liquids and of soap bubbles. Surface tension is responsible for the shape of the interface between two immiscible liquids.
Since these intermolecular forces vary depending on the nature of the liquid eg. Surface tension is responsible for the ability of some solid objects to float on the surface of a liquid. Surface tension is involved in the process of digestion.
Surface tension property of a liquid surface displayed by its acting as if it were a stretched elastic membrane.
 Surface Tension Definition Causes Measurement Formula Video Lesson Transcript Study Com
Surface Tension Definition Causes Measurement Formula Video Lesson Transcript Study Com
 Surface Tension Introduction To Chemistry
Surface Tension Introduction To Chemistry
 Chemistry 8 2b Properties Of Liquids Surface Tension And Capillary Action Youtube
Chemistry 8 2b Properties Of Liquids Surface Tension And Capillary Action Youtube
 Surface Tension What Is It How Does It Form What Properties Does It Impart Youtube
Surface Tension What Is It How Does It Form What Properties Does It Impart Youtube
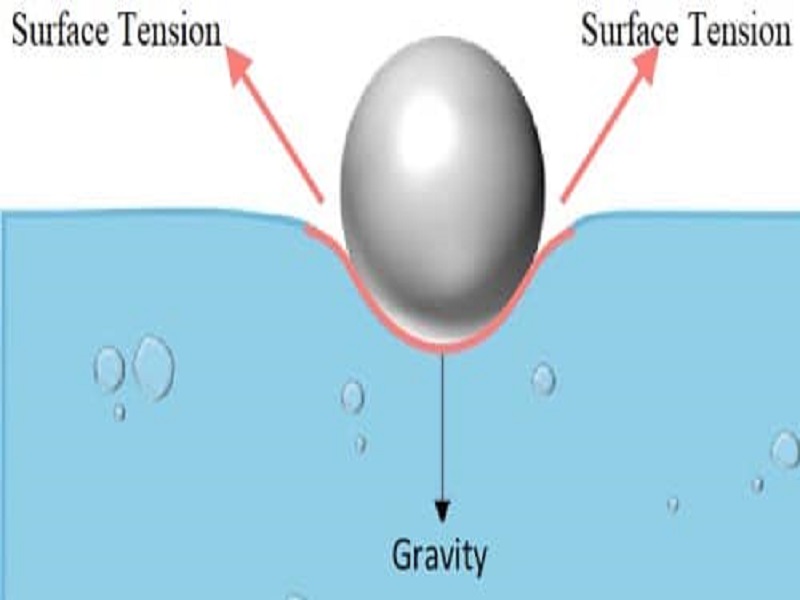 10 Surface Tension Examples In Daily Life Studiousguy
10 Surface Tension Examples In Daily Life Studiousguy
 Surface Tension Definition Measurement
Surface Tension Definition Measurement
 Surface Tension Chapter 5 The Water Molecule And Dissolving Middle School Chemistry
Surface Tension Chapter 5 The Water Molecule And Dissolving Middle School Chemistry
