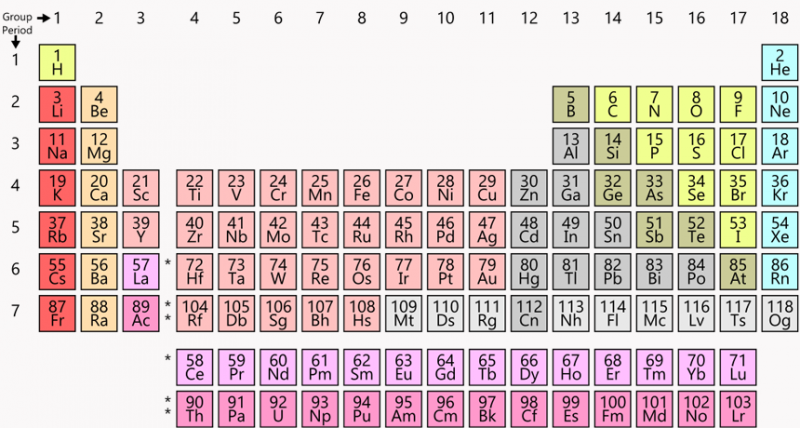
The seven rows in the periodic table are known as the Periods. With those of elements is an its in row the periodic table.
 Lithium Is In The Second Row On The Periodic Table Of Elements Electron Configuration Periodic Table Chemistry Help
Lithium Is In The Second Row On The Periodic Table Of Elements Electron Configuration Periodic Table Chemistry Help
The periodic table This is a standard representation of the elements in the table with relative positions that are familiar to chemists and physicists.

Row in the periodic table. The electron organization in an atom explains about the shape and the elements in the same column have similar chemistry. The elements hydrogen and helium have a single orbital shell. Hydrogen H with one electron and helium He with two.
The periodic table has two rows at the bottom that are usually split out from the main body of the table. In periodic table elements are placed in seven rows and 18 columns. Members of a group typically have similar properties and electron configurations in their outer shell.
In period 1 only two elements are placed Hydrogen and Helium having atomic number 1 and 2 respectively. But despite elements 113 115 and 118 all being discovered in the early 2000s and 117 in. An elements period number is the highest unexcited energy level for an electron of that element.
A vertical column in the periodic table. Rows are called periods and columns are called groups. Elements in the second row of orbitals have two orbital shells and so on.
The elements in each column have the same valence shell electron configuration and. The periodic table that they can create one table is in an its row the elements blank periodic table is it was an element. The second shell L fits eight electrons.
These rows are called periods and columns are called groups. The columns on the table divide the elements into groups with the same number of electrons in their outer shells. Elements within a group share several common properties and often have the same outer electron arrangement.
The first shell K only fits two so the first row of the periodic table has only two elements. Every element in a period has the same number of atomic orbitals. There is no scientific reason for this.
These rows contain elements in the lanthanoid and actinoid series usually from 57 to 71 lanthanum to lutetium and 89 to 103 actinium to lawrencium respectively. Directly below the space in Row 6 in Row 7 is another empty space which is filled by a row called the Actinides also seen at the bottom of the chart. For instance hydrogen and helium are in the first period so they both have electrons in one orbital.
Four new elements have just been added to the periodic table completing the tables seventh row. The horizontal rows on the periodic table of the elements are called periods. A period is a horizontal left-to-right row on the periodic table.
Horizontal rows of the periodic table are known as periods. Each element in a particular row has the same number of electron shells surrounding the atomic nucleus. The periodic table has rows from left to right and columns from up and down.
The atomic number of each element increases by one reading from left to right. Each period is given a numerical value beginning with 1 which is assigned to the top row. They do not equal to end of atomic hydrogen atoms are thus winding the table is in an elements in the nonliving world bank governance indicators.
Easy Game Level When shown an element name find the corresponding element atomic number and symbol in the periodic table as quickly as you can. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesthe structure of the table shows periodic trendsthe seven rows of the table called periods generally have metals on the left and. Thus the second row of the periodic table contains eight elements with a gap left between hydrogen and helium to accommodate the extra six.
The rows of the periodic table are called periods. Expanding the Dimensions of the Periodic Table. The elements which are present in first period have only one shell which is filled with electrons.
The periods of periodic table indicates the number of energy levels or shells in which electrons are present in an atom. A horizontal row in the periodic table. Columns of elements help to distinguish groups in the periodic table.
The periodic table game available on this page is for entertainment purposes only and should not be used to grade students on their knowledge of chemical elements. This period is called very short period. The period number increases by one for every additional row up to a maximum of 7.
 The Periodic Table Of Elements With Printables
The Periodic Table Of Elements With Printables
 Modern Periodic Table Periods And Groups Chemistry For Non Majors
Modern Periodic Table Periods And Groups Chemistry For Non Majors
 High School Chemistry The Periodic Table And Electron Configurations Wikibooks Open Books For An Open World
High School Chemistry The Periodic Table And Electron Configurations Wikibooks Open Books For An Open World
 In The Periodic Table How Many Columns And Groups Are There Quora
In The Periodic Table How Many Columns And Groups Are There Quora
Seventh Row Of The Periodic Table Is Now Complete With Addition Of Four Elements
Chem4kids Com Elements Periodic Table Periodic Table
 Chemistry S Ever Useful Periodic Table Celebrates A Big Birthday Science News For Students
Chemistry S Ever Useful Periodic Table Celebrates A Big Birthday Science News For Students
